T Regulatory Cell Responses in Toxoplasma-Infected Muscles
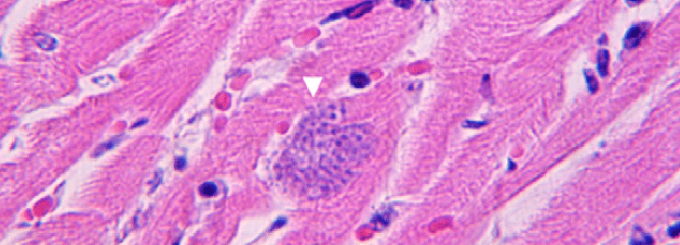
A cluster of Toxoplasma gondii cells in a section of the heart muscle as seen under a microscope.
Co-Investigator: Kirkwood Personius, PT, PhD
Principal Investigator: Elizabeth Wohlfert, PhD, department of microbiology and immunology
Funding Agency: National Institute of Allergy and Infectious Disease
Awarded: November 2021
Abstract: After the tissue is infected, the invading pathogen must be controlled and the tissue must be repaired or else long-term morbidity and mortality will ensue. Toxoplasma gondii infections result in a non-resolving chronic infection with tissue cysts residing preferentially in skeletal muscle and the central nervous system. In contrast to the brain, little is known about the immune response and immune-regulation in the infected muscle. Foxp3-expressing CD4 regulatory T cells (Tregs) play a key role in controlling the immune response to infection and aid in tissue repair. Yet, we have found during chronic T. gondii infection muscle Tregs become pathogenic and cause inflammation instead of preventing it. We have shown these Tregs are Th1-polarized but do not produce IFN-y.
Tregs in T. gondii-infected muscle produces low levels of amphiregulin, a cytokine critical in repairing immune-mediated pathology. We showed that Toxoplasma-induced immunopathology in muscle can be ameliorated by recombinant treatment with amphiregulin in chronically infected mice and returning function to the tissue. However, it is unclear how amphiregulin mediates this effect.
The overarching hypothesis for this proposal is that T-bet expression in skeletal muscle Tregs drives this pathogenic function by dampening the production of key factors associated with normally suppressive Treg function. This hypothesis will be addressed in the following specific aims: (1) identify how T-bet drives the pathogenic function of Tregs in infected muscle and (2) identify the relevant cellular targets of amphiregulin in infected muscle during Toxoplasma infection. How a chronic infection alters the highly orchestrated immune-mediated regeneration of muscle is still poorly understood. Loss of muscle mass predicts reduced quality of life and often increased morbidity for chronically ill patients and so a better understanding of what drives this process is needed for directing therapeutic interventions.