- Rehabilitation Science >
- Research and Facilities >
- Funded Research >
- ASPIRE: Assessing non-invasive Spinal stimulation and PT/OT for motor Improvement Response with ExaStim
ASPIRE: ExaStim Upper Limb Pivotal Clinical Validation Study
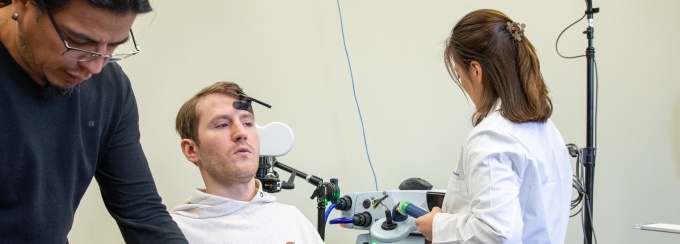
ASPIRE: Assessing non-invasive Spinal stimulation and PT/OT for motor Improvement Response with ExaStim
Lead Principal Investigator: Hang JIn Jo, PT, PhD
Funding Agency: Niche Biomedical Incorporated
Awarded: December 2022
Abstract: This clinical trial study is intended to improve and restore upper limb motor functions for individuals with paralysis using ExaStim. ExaStim is expected to provide more effective treatment relative to the current U.S. standard of care for the irreversibly debilitating condition of paralysis due to spinal cord injury.
ExaStim is a portable, noninvasive medical device that delivers patient specific, transcutaneous spinal cord stimulation. ExaStim consists of a multi-electrode pad, return electrodes, a stimulation device and mobile user interfaces for clinical professionals and patients. The device is worn and/or carried by the patient while in use. The multi-electrode pad allows for transcutaneous stimulation at multiple spatial locations along the spinal column. The pad consists of 16 neurostimulation hydrogel surface electrodes, composed of the same biocompatible materials used in standard neurostimulation surface electrodes with adhesive to adhere to the patient’s back of neck. The multi-electrode array connects to the stimulation device via a wired cable

